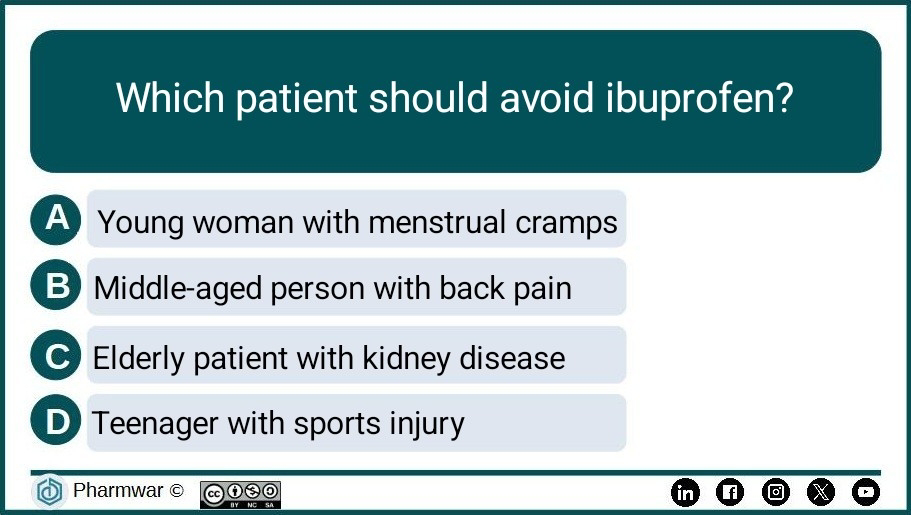
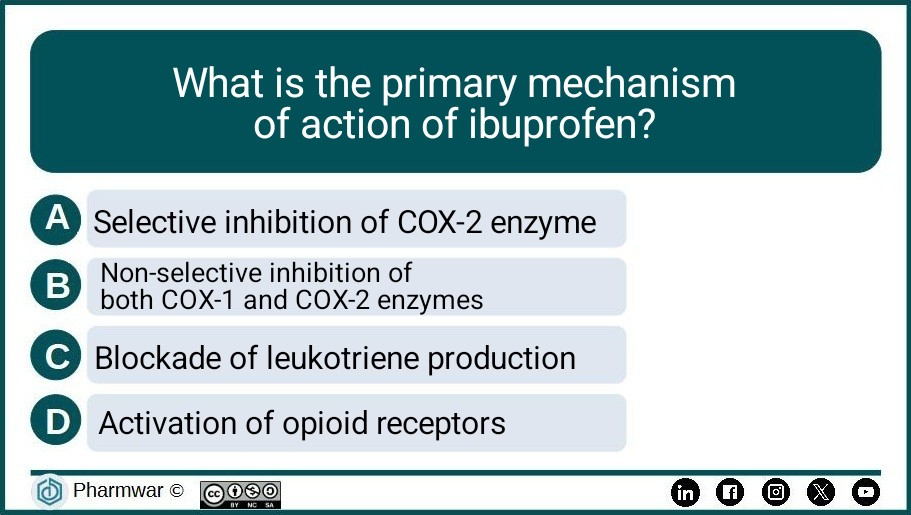
Pharma Quiz 7

Answer: b) N-(4-hydroxyphenyl)acetamide. Description: This is the IUPAC (International Union of Pure and Applied Chemistry) chemical name for Paracetamol. It highlights its structure as an acetamide derivative with a hydroxyl group at the para position of the phenyl ring, which is crucial for its pharmacological activity.